
Welcome to OnlineConversion.com
The SI
SI
The International System of Units (abbreviated SI from the French phrase, Système International d'Unités) is the most widely used system of units. It is the most common system for everyday commerce in the world, and is almost universally used in the realm of science.
In 1960, the SI was developed from a subset of the existing Metre-Kilogram-Second systems of units (MKS), rather than the older Centimetre-Gram-Second system (CGS). Various new units were created at that time. The SI is not static, it is a living set of standards where units are created and definitions are modified with international agreement. The SI authority describes SI as the modern metric system where modern means post-1960. It is common for people to describe non-SI units as 'metric', although there is no authority that maintains a current definition of metric system other than SI.
With a few exceptions (such as draught beer sales in the United Kingdom) the system is legally being used in every country in the world and many countries do not maintain definitions of other units. In the United States the use of SI is increasing but is still limited. In the United Kingdom, conversion to metric units is official policy but not yet complete. Those countries that still give recognition to non-SI units (e.g. the US and UK) have defined many of the modern units in terms of SI units.
History
The metric system was officially adopted in France after the French Revolution. During the history of the metric system a number of variations have evolved and their use spread around the world replacing many traditional measurement systems.
By the end of World War II a number of different systems of measurement were still in use throughout the world. Some of these systems were metric system variations whilst others were based on the Imperial and American systems. It was recognised that additional steps were needed to promote a worldwide measurement system. As a result the 9th General Conference on Weights and Measures (CGPM), in 1948, asked the International Committee for Weights and Measures (CIPM) to conduct an international study of the measurement needs of the scientific, technical, and educational communities.
Based on the findings of this study, the 10th CGPM in 1954 decided that an international system should be derived from six base units to provide for the measurement of temperature and optical radiation in addition to mechanical and electromagnetic quantities. The six base units recommended were the metre, kilogram, second, ampere, Kelvin degree (later renamed the kelvin), and the candela. In 1960, the 11th CGPM named the system the International System of Units, abbreviated SI from the French name: Le Système International d'Unités. The seventh base unit, the mole, was added in 1970 by the 14th CGPM.
The International System is now either obligatory or permissible throughout the world. It is administered by the standards organisation: the Bureau International des Poids et Mesures (International Bureau of Weights and Measures).
Cultural issues
The swift worldwide adoption of the metric system as a tool of economy and everyday commerce was based mainly on the lack of customary systems in many countries to adequately describe some concepts, or as a result of an attempt to standardize the many regional variations in the customary system. International factors also affected the adoption of the metric system, as many countries increased their trade. Scientifically, it provides ease when dealing with very large and small quantities because it lines up so well with our decimal numeral system.
Cultural differences can be represented in the local everyday uses of metric units. For example, bread is sold in one-half, one or two kilogram sizes in many countries, but you buy them by multiples of one hundred grams in the former USSR. In some countries, the informal cup measurement has become 250 mL, and prices for items are sometimes given per 100 g rather than per kilogram. A profound cultural difference between physicists and engineers, especially radio engineers, existed prior to the adoption of the metre-kilogram-second (MKS) system and hence its descendent, SI. Engineers work with volts, amperes, ohms, farads, and coulombs, which are of great practical utility, while the centimetre-gram-second (CGS) units, which are fine for theoretical physics can be inconvenient for electrical engineering usage and are largely unfamiliar to householders using appliances rated in volts and watts.
Non-scientific people should not be put off by the fine-tuning that has happened to the metric base units over the past 200 years, as experts have tried frequently to refine the metric system to fit the best scientific research (e.g. CGS to MKS to SI system changes or the invention of the Kelvin scale). These changes do not affect the everyday use of metric units. The presence of these adjustments has been one reason advocates of the U.S. customary units had used against metrication; these customary units, however, are nowadays defined in terms of SI units, thus any difference in the definition of the SI units results in a difference of the definition of the customary units.
SI writing style
- Symbols are written in lower case, except for symbols derived from the name of a person. For example, the unit of pressure is named after Blaise Pascal, so its symbol is written "Pa" whereas the unit itself is written "pascal". The one exception is the litre, whose original abbreviation "l" is dangerously similar to "1". The NIST recommends that "L" be used instead, a usage which is common in the U.S., Canada and Australia, and has been accepted as an alternative by the CGPM. The cursive "l" is occasionally seen, especially in Japan, but this is not currently recommended by any standards body. For more information, see Litre.
- Symbols are written in singular form: i.e. "25 kg", not "25 kgs". Pluralization would be language dependent; "s" plurals (as in French and English) are particularly undesirable since "s" is the symbol of the second.
- Symbols do not have an appended period (.).
- It is preferable to write symbols in upright Roman type (m for metres, L for litres), so as to differentiate from the italic type used for mathematical variables (m for mass, l for length).
- A space should separate the number and the symbol, e.g. "2.21 kg", "7.3×102 m2", "22 °C". Exceptions are the symbols for plane angular degrees, minutes and seconds (°, ' and "), which are placed immediately after the number with no intervening space.
- Spaces should be used to group decimal digits in threes, e.g. 1 000 000 or 342 142 (in contrast to the commas or dots used in other systems, e.g. 1,000,000 or 1.000.000).
- The 10th resolution of CGPM in 2003 declared that "the symbol for the decimal marker shall be either the point on the line or the comma on the line". In practice, the full stop is used in English, and the comma in most other European languages.
- Symbols for derived units formed from multiple units by multiplication are joined with a space or centre dot (·), e.g. N m or N·m.
- Symbols formed by division of two units are joined with a solidus (/), or given as a negative exponent. For example, the "metre per second" can be written "m/s", "m s-1", "m·s-1" or
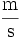 . A solidus should not be used if the result is ambiguous, i.e. "kg·m-1·s-2" is preferable to "kg/m/s2".
. A solidus should not be used if the result is ambiguous, i.e. "kg·m-1·s-2" is preferable to "kg/m/s2".
Spelling variations
- Several nations, notably the United States, typically use the spellings 'meter' and 'liter' instead of 'metre' and 'litre' in keeping with standard American English spelling. In addition, the official US spelling for the SI prefix 'deca' is 'deka'.
- The unit 'gram' is also sometimes spelled 'gramme' in English-speaking countries other than the United States, though that is an older spelling and use is declining.
Units
SI consists of seven base units and several derived units, together with a set of prefixes.
Base units
The following are the fundamental units from which all others are derived, they are dimensionally independent. The definitions stated below are widely accepted.
| SI Base units | ||||
|---|---|---|---|---|
| Name | Symbol | Quantity | Definition | |
| kilogram | kg | Mass | The unit of mass is equal to the mass of the international prototype kilogram (a platinum-iridium cylinder) kept at the Bureau International des Poids et Mesures (BIPM), Sèvres, Paris (1st CGPM (1889), CR 34-38). Note that the kilogram is the only base unit with a prefix; the gram is defined as a derived unit, equal to 1/1000 of a kilogram; prefixes such as mega are applied to the gram, not the kg; e.g. Gg, not Mkg. It is also the only unit still defined by a physical prototype instead of a measurable natural phenomenon (see the kilogram article for an alternate definition). | |
| second | s | Time | The unit of time is the duration of exactly 9 192 631 770 periods of the radiation corresponding to the transition between two hyperfine levels of the ground state of the caesium-133 atom at a temperature of 0 K (13th CGPM (1967-1968) Resolution 1, CR 103). | |
| metre | m | Length | The unit of length is equal to the length of the path traveled by light in a vacuum during the time interval of 1/299 792 458 of a second (17th CGPM (1983) Resolution 1, CR 97). | |
| ampere | A | Electrical current | The unit of electrical current is the constant current which, if maintained in two straight parallel conductors, of infinite length and negligible cross-section, placed 1 metre apart in a vacuum, would produce a force between these conductors equal to 2×10 -7 newtons per metre of length (9th CGPM (1948) Resolution 7, CR 70). | |
| kelvin | K | Thermodynamic temperature | The unit of thermodynamic temperature (or absolute temperature) is the fraction 1/273.16 (exactly) of the thermodynamic temperature at the triple point of water (13th CGPM (1967) Resolution 4, CR 104). | |
| mole | mol | Amount of substance | The unit of amount of substance is the amount of substance which contains as many elementary entities as there are atoms in 0.012 kilograms of pure carbon-12 (14th CGPM (1971) Resolution 3, CR 78). (Elementary entities may be atoms, molecules, ions, electrons, or particles.) It is approximately equal to 6.02214199×1023 units (Avogadro's number). | |
| candela | cd | Luminous intensity | The unit of luminous intensity is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540×1012 hertz and that has a radiant intensity in that direction of 1/683 watt per steradian (16th CGPM (1979) Resolution 3, CR 100). | |
Dimensionless derived units
The following SI units are actually dimensionless ratios, formed by dividing two identical SI units. They are therefore considered by the BIPM to be derived. Formally, their SI unit is simply the number 1, but they are given these special names, for use whenever the lack of a unit might be confusing.
| SI dimensionless units | ||||
|---|---|---|---|---|
| Name | Symbol | Quantity | Definition | |
| radian | rad | Angle | The unit of angle is the angle subtended at the centre of a circle by an arc of the circumference equal in length to the radius of the circle. There are 2π radians in a circle. | |
| steradian | sr | Solid angle | The unit of solid angle is the solid angle subtended at the centre of a sphere of radius r by a portion of the surface of the sphere having an area r2. There are 4π steradians on a sphere. | |
Derived units with special names
Base units can be put together to derive units of measurement for other quantities. Some have been given names.
| SI derived units with special names | ||||
|---|---|---|---|---|
| Name | Symbol | Quantity | Expressed in base units | |
| hertz | Hz | Frequency | s-1 | |
| newton | N | Force | m·kg·s-2 | |
| joule | J | Energy | N m = m2·kg·s-2 | |
| watt | W | Power | J/s = m2·kg·s-3 | |
| pascal | Pa | Pressure, Stress | N/m2 = m-1·kg·s-2 | |
| lumen | lm | Luminous flux | cd·sr = m2·m-2·cd = cd | |
| lux | lx | Illuminance | lm/m2 = m2·m-4·cd = m-2·cd | |
| coulomb | C | Electric charge or flux | s·A | |
| volt | V | Electrical potential difference | W/A = J/C = m2·kg·s-3·A-1 | |
| ohm | Ω | Electric resistance | V/A = m2·kg·s-3·A-2 | |
| farad | F | Electric capacitance | C/V = m-2·kg-1·s4·A2 | |
| weber | Wb | Magnetic flux | m2·kg·s-2·A-1 | |
| tesla | T | Magnetic flux density | Wb/m2 = kg·s-2·A-1 | |
| henry | H | Inductance | Wb/A = m2·kg·s-2·A-2 | |
| siemens | S | Electric conductance | Ω-1 = m-2·kg-1 s3·A2 | |
| becquerel | Bq | Radioactivity (decays per unit time) | s-1 | |
| gray | Gy | Absorbed dose (of ionising radiation) | J/kg = m2·s-2 | |
| sievert | Sv | Equivalent dose (of ionising radiation) | J/kg = m2·s-2 | |
| katal | kat | Catalytic activity | mol/s = s-1·mol | |
| degree Celsius | °C | Thermodynamic temperature | K (0 °C = 273.15 K, 0 K = -273.15 °C) | |
| molarity | M | Concentration | mol/L (mols of substance/liters of solution) | |
Non-SI units accepted for use with SI
The following units are not SI units but are "accepted for use with the International System."
| Non-SI units accepted for use with SI | ||||
|---|---|---|---|---|
| Name | Symbol | Quantity | Equivalent SI unit | |
| minute | min | time | 1 min = 60 s | |
| hour | h | time | 1 h = 60 min = 3600 s | |
| day | d | time | 1 d = 24 h = 1440 min = 86400 s | |
| degree of arc | ° | angle | 1° = (π/180) rad | |
| minute of arc | ' | angle | 1' = (1/60)° = (π/10800) rad | |
| second of arc | " | angle | 1" = (1/60)' = (1/3600)° = (π/648000) rad | |
| litre | l or L | volume | 0.001 m3 | |
| tonne | t | mass | 1 t = 103 kg | |
|
Non-SI units not formally adopted by the CGPM |
||||
| neper, field quantity | Np | ratio (dimensionless) | LF = ln(F/F0) Np | |
| neper, power quantity | Np | ratio (dimensionless) | LP = 1/2 ln(P/P0) Np | |
| bel, field quantity | B | ratio (dimensionless) | LF = 2 log10(F/F0) B | |
| bel, power quantity | B | ratio (dimensionless) | LP = log10(P/P0) B | |
|
Non-SI units with values obtained only by experiment |
||||
| electronvolt | eV | energy | 1 eV = 1.60217733 (49) × 10-19 J | |
| atomic mass unit | u | mass | 1 u = 1.6605402 (10) × 10-27 kg | |
| astronomical unit | AU | length | 1 AU = 1.49597870691 (30) × 1011 m | |
|
Non-SI units whose use is not encouraged |
||||
| nautical mile | length | 1 nautical mile = 1852 m | ||
| knot | speed | 1 knot = 1 nautical mile per hour = (1852/3600) m/s | ||
| are | a | area | 1 a = 1 dam2 = 100 m2 | |
| hectare | ha | area | 1 ha = 100 a = 10000 m2 | |
| bar | bar | pressure | 1 bar = 105 Pa | |
| a*ngström, angstrom | A* | length | 1 A* = 0.1 nm = 10-10 m | |
| barn | b | area | 1 b = 10-28 m2 | |
SI prefixes
SI defines a number of SI prefixes to be used with the units: these combine with any unit name to give subdivisions and multiples. For example, the prefix kilo denotes a multiple of a thousand, so the kilometre is 1000 metres, the kilogram 1000 grams, and so on. The prefixes are never combined; a millionth of a kilogram is a milligram, and not a 'microkilogram'.
The following SI prefixes can be used to prefix any of the above units to produce a multiple or submultiple of the original unit. This includes the degree Celsius (e.g. "1.2 m°C"); however, to avoid confusion, prefixes are not used with the time-related unit symbols min (minute), h (hour), d (day). They are not recommended for use with the angle-related symbols ° (degree), ' (minute of arc), and " (second of arc), but for astronomical usage, they are sometimes used with seconds of arc.
| 10n | Prefix | Symbol | Short scale | Long scale | Decimal equivalent |
|---|---|---|---|---|---|
| 1024 | yotta | Y | Septillion | Quadrillion | 1 000 000 000 000 000 000 000 000 |
| 1021 | zetta | Z | Sextillion | Trilliard (thousand trillion) | 1 000 000 000 000 000 000 000 |
| 1018 | exa | E | Quintillion | Trillion | 1 000 000 000 000 000 000 |
| 1015 | peta | P | Quadrillion | Billiard (thousand billion) | 1 000 000 000 000 000 |
| 1012 | tera | T | Trillion | Billion | 1 000 000 000 000 |
| 109 | giga | G | Billion | Milliard (thousand million) | 1 000 000 000 |
| 106 | mega | M | Million | 1 000 000 | |
| 103 | kilo | k | Thousand | 1 000 | |
| 102 | hecto | h | Hundred | 100 | |
| 101 | deca, deka | da | Ten | 10 | |
| 100 | none | none | One | 1 | |
| 10-1 | deci | d | Tenth | 0.1 | |
| 10-2 | centi | c | Hundredth | 0.01 | |
| 10-3 | milli | m | Thousandth | 0.001 | |
| 10-6 | micro | µ | Millionth | 0.000 001 | |
| 10-9 | nano | n | Billionth | Milliardth | 0.000 000 001 |
| 10-12 | pico | p | Trillionth | Billionth | 0.000 000 000 001 |
| 10-15 | femto | f | Quadrillionth | Billiardth | 0.000 000 000 000 001 |
| 10-18 | atto | a | Quintillionth | Trillionth | 0.000 000 000 000 000 001 |
| 10-21 | zepto | z | Sextillionth | Trilliardth | 0.000 000 000 000 000 000 001 |
| 10-24 | yocto | y | Septillionth | Quadrillionth | 0.000 000 000 000 000 000 000 001 |
Obsolete metric prefixes
The following metric prefixes are no longer in use: myria-, myrio-, and any double prefixes such as those formerly used in micromicrofarads, hectokilometres, millimicrons.
This article is available under the terms of the GNU Free Documentation License. Original article can be found here.